

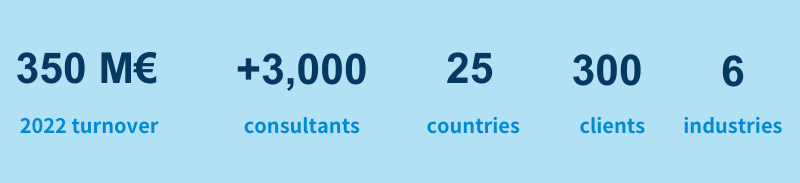
ALTEN: the European consulting leader in the Life Sciences industry
By leveraging a deep understanding of the sector’s complexities along with cutting-edge technological expertise, ALTEN has been instrumental in accelerating the development of innovative healthcare solutions. Our collaboration with leading pharmaceutical companies has led to the faster time-to-market of safe and effective medication. Our involvement in biomedical technology has facilitated the creation of advanced medical devices, improving patient outcomes and healthcare delivery. ALTEN’s commitment to excellence and innovation not only drives progress in the Life Sciences field but also exemplifies our role as a key partner for our customers.
OUR EXPERTISE
Automation
Automation refers to the use of various technologies and systems to perform tasks that were traditionally done manually, increasing efficiency, accuracy, and reproducibility.
Automation plays a pivotal role in streamlining research and development processes, enhancing the precision of experimental results, and accelerating the pace of discovery in biotechnology, pharmaceutical manufacturing, and quality control.
By minimising human error and optimising resource use, automation significantly contributes to the industry’s ability to innovate and bring new products to market more rapidly and safely, ultimately improving patient care and public health outcomes.

Computerised System Validation
CSV is a critical process designed to ensure that each computer-based system operates within a set parameter of reliability, accuracy, and predictiveness.
This protocol is paramount in laboratories, research centers, and pharmaceutical companies, where it guarantees that software and computer systems are performing as intended, especially in environments where data integrity is crucial for research, development, and regulatory compliance.
Through CSV, the Life Sciences sector can uphold the highest standards of quality control, ensuring that the technologies they rely on meet stringent regulatory requirements and contribute effectively to health and safety outcomes.

Manufacturing
Manufacturing refers to the complex and highly regulated process of producing pharmaceuticals, biotechnology products, and medical devices that are safe, effective, and of high quality.
This involves a series of precise steps, including the synthesis of active pharmaceutical ingredients, formulation into final products, and thorough packaging and labeling.
Manufacturing within the Life Sciences industry must adhere to Good Manufacturing Practices (GMP), ensuring that products are consistently produced and controlled according to quality standards. This critical phase in the product lifecycle not only demands stringent adherence to regulatory requirements but also necessitates advanced technological support and meticulous oversight to maintain the integrity, efficacy, and safety of life-saving health products.

Quality Assurance
Quality Assurance (QA) is a systematic and proactive process aimed at ensuring that all products or services meet consistent, high-quality standards.
Quality Assurance is about preventing flaws in the development and manufacturing processes (while Quality Control focuses on the end product). This involves setting stringent operational procedures and standards, conducting regular audits and reviews, and ensuring that procedures are followed correctly at every stage of product development, manufacturing, and distribution.
QA is critical in the Life Sciences sector, as it helps maintain the safety, efficacy, and reliability of pharmaceuticals, biotech products, and medical devices, directly impacting patient health and safety. Quality Assurance, therefore, serves as the foundation for trust in Life Sciences, ensuring that every product released not only complies with regulatory requirements but also meets the industry’s high standards and expectations for quality.

Quality Control
Quality Control (QC) is an essential process that ensures products meet the required safety, efficacy, and quality standards before they reach the market.
This involves rigorous testing and evaluation of pharmaceuticals, biotechnology products, and medical devices at various stages of production to identify and rectify any defects or deviations from the established standards.
Quality Control procedures are critical in maintaining the integrity of the production process, from raw material inspection to the final product release, ensuring that every item complies with regulatory requirements and meets the industry’s high standards of quality and reliability. Through these meticulous QC processes, companies in the Life Sciences sector can guarantee that their products are consistently safe and effective for consumer use, ultimately safeguarding public health.

Regulatory Affairs
Regulatory Affairs in the life sciences sector is a discipline that focuses on ensuring compliance with all the regulatory standards set forth by governing bodies.
Professionals in this field play a critical role in the entire lifecycle of a product, from its initial design, through clinical trials, to its market release and beyond. They are responsible for preparing detailed documentation and applications that are submitted to regulatory agencies to obtain the necessary approvals for new drugs, devices, and treatments.
Additionally, Regulatory Affairs experts monitor the product’s compliance with regulatory requirements post-market launch and adapt to any legislative changes that may occur. This ensures that products not only reach the market efficiently but also remain available and maintain the highest safety and efficacy standards for consumer use.

HOT TOPICS IN THE LIFE SCIENCES INDUSTRY IN 2024
Synergising disciplines for biotech innovations
Cross-disciplinary collaborations drive a broad spectrum of biotech innovations, accelerating medical advancements for safer, more precise treatments. Highlights include AI-powered CRISPR for gene editing, portable diagnostics through lab-on-a-chip technology, RNA therapies targeting HIV and cancer, bioprinting and tissue engineering for regenerative medicine, and stem cell applications broadening drug testing and disease models.
Additionally, predictive modelling boosts drug development efficiency, while personalised medicine customises treatment based on genetic profiles. This collaborative ecosystem forecasts a trend towards strategic mergers and acquisitions, reflecting the impactful transformation of scientific discoveries into clinical and societal benefits.

Integrating emerging technologies into manufacturing
AI-enabled intelligent automation and advanced analytics are reshaping pharmaceutical manufacturing, making it agile, eco-friendly, and focused on patients’ needs. Real-time data and analytics allow for immediate operational decisions and improvements, addressing supply chain and talent shortages. Digital twins virtually simulate manufacturing facilities to optimise processes and enable predictive maintenance, reducing time-to-market for drugs.
These advances support the shift from traditional batch methods to continuous manufacturing, further e nhancing efficiency while cutting waste. Meanwhile, flexible production technologies like single-use bioreactors, modular systems, and 3D printing are advancing personalised medicine by enabling the production of small, tailored drug batches.

Aligning regulations with technological advances
Regulatory evolutions underscore a global shift towards more adaptive and patient-centric regulatory frameworks, ensuring safety and efficacy in the face of technological advances.
The European Union is debating reforms to tackle potential medicine shortages, fight antimicrobial resistance, and improve access to medicines. These discussions are also addressing supply disruptions under new medical device and in vitro diagnostics regulations.
